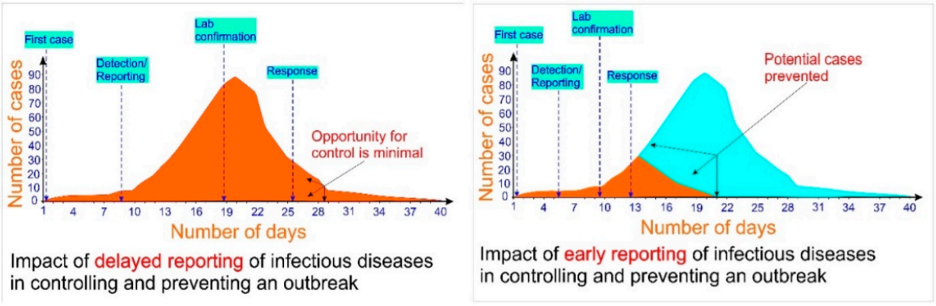
COVID-19 has created chaos worldwide and its new variants are continuously spreading across the borders. The current diagnostic approach for COVID-19 is mainly focused on the real-time reverse transcription-polymerase chain reaction (rRT-PCR), a golden standard molecular technique. This rRT-PCR technique requires sophisticated laboratory equipment that is often located at the central laboratory. Therefore, the time required to obtain the results can be up to 2 days because of time consumed in collecting samples, packing (storage), shipment of the clinical specimens under controlled temperature, multi-step sample preparation protocols, and laboratory testing. In this scenario, a point-of-care (PoC) device is an alternative robust solution that is crucial and urgently needed. PoC testing is rapid, robust, and cost-efficient that can be used on-site and in the field with minimal training. Rapid diagnostics will have significant impact on the early detection of infectious diseases and in controlling an outbreak (Fig. 1). Loop-mediated isothermal amplification (LAMP) is a novel nucleic acid amplification technique. LAMP amplifies DNA with high specificity, efficiency, and rapidity under isothermal conditions at a constant temperature of 65 °C
Figure 1 Impact of rapid diagnostic technique in controlling and preventing an outbreak
Table 1 Comparison of key features of PCR and LAMP (Nguyen et al.)
|
PCR |
LAMP |
|---|---|
|
Thermal cycling (Multiple heating and cooling cycle; hence, bulky and cumbersome). |
Isothermal and continuous amplification (Smaller, simpler, hence portable). |
|
Always requires sample concentration and purification (Time-consuming). |
LAMP assay offers simplified sample preparation steps and one-step detection. |
|
Multi-step complicated protocols that requires a skilled technician. |
Simplified and faster protocols that require minimal training. |
|
Inhibitors hinder the reaction. |
Tolerate inhibitors and more stable. |
|
Established technique. |
Emerging and highly potential novel technology |
Therefore, we believe, LAMP could be a potential candidate for the point-of-care device application in the detection of COVID-19.
References:
- Nguyen et al. Micromachines 2020, 11(3), 306; https://doi.org/10.3390/mi11030306
- World Health Organization (WHO). Outbreak Investigation. https://www.who.int/hac/techguidance/training/outbreak%20investigation_en.pdf